| |
After 10 years of collaborative interdisciplinary research, Bio-Systems and Micro-Mechanics (BioSyM) IRG has produced groundbreaking technologies, scientific discoveries, and a rich pool of talent with diverse backgrounds. Join us at this networking event and find exciting ideas, novel devices and systems, and meet the people spearheading the efforts.
Keynote Talks:
Prof. Roger Kamm, MIT, & Prof. Hanry Yu, NUS
Overview of research and innovation:
Microfluidics; Optical Instrumentation; Bio-Imaging; Bio-Film; Stem Cells; and Cancer
Posters / Demonstrations & Networking Dinner @ CREATE Enterprise Wing Lounge (2nd Floor)
Signup! @ https://tinyurl.com/biosym10
BioSyM Seminar Series 2018
Speaker |
Title of the Talk |
Date & Time |
Venue |
| Prof. Michael BIRNBAUM (MIT) |
Decoding T and NK cell recognistion |
21/January/2019 |
CREATE Enterprise Level 5, Perseverance Rooms, UTown |
| Dr.Bernard Willems (AYOXXA) |
A New Technology for Multiplexed Protein Analysis |
10/Dec/2018 |
| Dr.Debasis Banik (SMART) |
Investigations of T Cell Receptor Quality Against Tumor Neoantigens |
26 / Nov / 2108 |
| Dr.Chen Zhi (Vice President, Orbbec Co Ltd) |
3D Sensing - Eyes of Artificial Intelligence |
9 / Nov / 2018 |
Dr.Lim Sei Hien (AIM Biotech)
|
Challenges of Working in a Start-Up |
5/Nov/2018 |
| Dr.Ali Asgar Bhagat (Exploit Technologies, A*STAR) |
|
22/October/2018 |
| Prof.Jongyoon Han (MIT) |
Electrokinetic molecular concentration: My journey |
2/October/2018 |
|
Dr.Eunice Yeap (SMART)
|
Drug Particle Engineering using Microfluidic Emulsion Crystallisation |
17/September/2018 |
| Dr.Arif Z Nelson (SMART) |
Designing with Rheological Complexity |
3/September/2018 |
| Dr. Younghoon Shin (SMART) |
Oblique scanning 2-photon light-sheet fluorescence microscopy for rapid volumetric imaging |
20/August/2018, 12 noon |
Genichi Tsuzawa (Director, RIKEN, Singapore)
|
RIKEN National Science Institute: An Overview |
30/July/2018, 12 noon |
| Dr.Levi Wood (Georgia Institute of Tech, USA) |
Systems Analysis of Neuroinflammation in Alzheimer's Disease and Traumatic Brain Injury |
9/July/2018, 3 pm |
CREATE THEATRETTE, UTown |
| Dr.Zhu Hai (SMART) |
Adaptive Optics for Stimulated Raman Scattering Imaging and Optical Tweezers |
18/June/2018, 12 noon |
CREATE Enterprise Level 5, Perseverance Rooms, UTown |
| Shang Menglin (NUS/SMART) |
Investigating elevated interstitial fluid pressure-enhanced drug resistance of human cancer cells |
4/June/2018, 12 noon |
| Dr.Dai Liang (SMART) |
Some physics problems in nanochannel-and nanopore-based technologies for DNA analysis |
21/May/2018, 12 noon |
| Charlotte BOUQUEREL (NUS/SMART) |
Isolation of Bladder Cancer Cells From Voided Urine in Relation To Patient Prognosis |
7/May/2018, 12 noon |
| Dr.Dawei Ding (SMART) |
Design of MucoadhesiveMicroparticlesfor Ocular Drug Delivery |
23/April/2018, 12 noon |
|
|
Understanding heterogeneity of human Mesenchymal Stem Cells (hMSCs) |
9/April/2018, 12 noon |
| Dr.Chew Su Chuen (SMART) |
The multifaceted nature of bacterial interactions that impact on community function, virulence and evolution |
26/March/2018, 12 noon |
|
|
Testing anti-angiogenic potency of novel compounds in a 3D microfluidic assay and in a Zebrafish model |
12th March 2018, 12 noon |
| Dr.Zhang Zhengyun (SMART) |
|
26th February 2018, 12 noon |
| Dr.Zhu Qingdi (SMART) |
Strain Sensitive stretchable mechanochromic hydrogel |
5th February 2018, 12 noon |
@ CREATE Theatrette, Level 2. 1 CREATE Way, CREATE Tower, Singapore 138602
BioSyM Seminar Series 2017
Speaker |
Title of the Talk |
Date & Time |
Venue |
| Dr.Kerwin (SMART) |
Precision Microfluidics for Customised Cell Isolation and Real-time Immuno-profiling from Whole Blood |
18th December 2017, 12 noon |
CREATE Enterprise Level 5, Perseverance Rooms, UTown |
| Dr.Sharon ONG (SMART) |
Integrating a Robotic Micropipette with an Optical Trap for T-cell Screening and Manipulation |
11th December 2017, 12 noon |
| Dr.Yin Lu (SMART) |
Application of spiral microchannel device in cell-based cartilage repair |
20th November 2017, 12 noon |
| Mr.Ng Ee Xien (SMART) |
Integrated Microfluidics for Protease Assay and Single Cell Analysis for Precision Medicine |
13th November 2017, 12 noon |
| Dr.Sharon Ong (SMART) |
(1) Automatic tracking of bacteria in early biofilm formation
(2) A robotic micropipette for cell manipulation |
6th November 2017, 12 noon |
| Dr.Smitha (SMART) |
Magnetic Resonance Relaxometry detection of early stage infection and Artemisinin resistant of Plasmodium falciparum |
23rd October 2017, 12 noon |
| Dr.Binu Kundukad (SMART) |
Mechanistic action of weak acid drugs on biofilms |
25th September 2017, 12 noon |
| Dr. Dipanjan Bhattacharya (SMART) |
Whole embryo 3D deformation map during Zebrafishearly embryogenesis |
11th September 2017, 12 noon |
| Dr.Abhinay Mishra (SMART) |
Synthesis and fabrication of polymer / nanohybridsfor biomedical applications |
28th August 2017, 12 noon |
| Dr.Agnes Miermont (SMART) |
Impact of osmotic pressure on cancer cell migration |
31st July 2017, 12 noon |
| Dr.Cristina Bertocchi (MBI, NUS) |
Nanoscale architecture and molecular mechanosensingof cadherin mediated adhesion |
17th July 2017, 4 pm |
| Ms Tee Ching Ann (NTU / SMART) |
Application of Biophysically Sorted Chondrocyte in Articular Cartilage Regeneration |
10th July 2017, 12 noon |
Prof.Krystyn Van Vliet (MIT/SMART) |
Nuts and Bolts of Academic Career Building |
27th June 2017, 12 noon |
CREATE Theatrette, Level 2, CREATE Tower @ UTown |
Prof.Sanjeev R. Inamdar (Karnatak Univ., India) |
Fluorescence Resonance Energy Transfer in quantum dot–dye systems |
19th June 2017, 12 noon |
CREATE Enterprise Level 5, Perseverance Rooms, UTown |
Prof.Harry ASADA (MIT/SMART) |
From IDEAS to successful RESEARCH PROPOSALS |
12th June 2017, 12 noon |
Dr.Andrea Ravasio (SMART) |
Mechanobiology of collectively migrating cells |
5th June 2017, 12 noon |
|
Mr. Chee Keong Kwok ( University of Würzburg)
|
Scalable suspension culture for the generation of billions of human induced pluripotent stem cells using single-use bioreactors |
19th May 2017, 12 noon |
Evan Tan (DBS, NUS / SMART) |
Role of Jag1 Extracellular Vesicles on Angiogenesis |
24th April 2017, 12 noon |
Prof.Zhang Li Feng (NTU) |
High-resolution RNA allelotypingalong the inactive X chromosome: evidence of RNA polymerase III in regulating chromatin configuration |
17th April 2017, 4 pm |
Prof.GE Ruowen (DBS, NUS) |
Targeting cell-surface GRP78 for anticancer therapy |
10th April 2017, 4 pm |
CREATE Theatrette, Level 2, CREATE Tower @ UTown |
Sharon Lee (NUS/SMART) |
On-Chip Elucidation of the Role of Monocytes in T cell Immunotherapy |
3rd April 2017, 12 noon |
BioSyM Meeting Room, Level 4, Enterprise Wing, 1 CREATE Way, University Town |
Yan Teck Ho (NUS / SMART) |
Probing the effect of the protein corona on nanoparticle permeability in cancer medicine |
27th March 2017, 12 noon |
Prof.Nanguang CHEN (NUS) |
Multi-dimensional high-resolution imaging of Zebrafish organs using line-scan focal modulation microscopy |
20th March 2017, 4 pm |
Prof Roger D. Kamm (SMART/MIT) |
Models for Human Disease: An Engineering Perspective |
6th March 2017, 4 pm |
CREATE Theatrette, Level 2, CREATE Tower @ UTown |
Prof Dahai LUO (LKC, NTU)
|
The Molecular Portraits of the Dengue Virus Replication Machinery |
27 February 2017, 4 pm |
BioSyM Meeting Room, Level 4, Enterprise Wing, 1 CREATE Way, University Town |
Prof.Evelyn KF YIM (MBI/NUS) |
Topographical Regulations on Cell Behaviors for Tissue Engineering Applications |
23 February 2017, 4 pm |
Prof. Anand ASUNDI (NTU) |
d’Biomager–A Quantitative Phase Imaging Add-on for Microscopes |
20 February 2017, 4 pm |
CREATE THEATRE Level 2, CREATE TOWER, 1, CREATE WAY, Singapore 138602 |
CREATE SYMPOSIUM / CREATE TOWER / 1 CREATE WAY / SINGAPORE 138602
16th January 2017
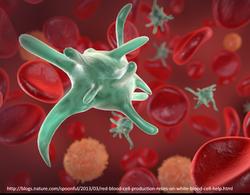
This symposium discusses the current state-of-art and opportunities for Singapore to become a world leader in cell manufacturing. Biological cell-based therapies offer the potential to improve human health, but require advanced manufacturing technologies and workforces to produce cells and cell-products safely and at scale. This symposium addresses challenges and opportunities in cell and cell-product manufacturing in Singapore and the US. This public symposium includes industry, academic, and government stakeholders in the research, innovation, and enterprise to produce cell products ranging from regenerative stem cells to cancer therapies to vaccines
BioSyM Focused Seminar Series
New Researchers Talk
10 October — 19 December 2016
Speaker |
Title of the Talk |
Date & Time |
Venue |
| Dr.Ajeetkumar Patil (SMART) |
Development of Miniature laser Induced Autofluorescence set up for in vivo Tissue Spectroscopy |
19th Dec 2016, Monday, 12-1 pm |
CREATE Enterprise Level 5, Perseverance Rooms, UTown |
| Dr.Kerwin Kwek (SMART) |
Determenistic lateral displacement methods for precision microfluidic separation of micro to nano-particles |
12th Dec 2016, Monday, 12-1 pm |
| Dr.Song Jiho (SMART) |
Quantitative assessment of circulating tumor cell extravasation in a 3D microvascular network system |
5th Dec 2016, Monday, 12-1 pm |
| Dr.Darwesh MK Aladin (SMART) |
E-cadherin's extracellular domains determine key epithelium mechanical phenotypes |
28th Nov 2016, Monday, 12-1 pm |
| Dr.Kimberly TAM (SMART) |
A nanoscaffold impregrnated with human wharton's jelly stem cells or its secretions improves healing of wounds |
21st Nov 2016, Monday, 12-1 pm |
| Dr.Dawei DING (SMART) |
Engineering of Biomemetic Materials Inspired by Jumbo Squid Sucker Ring Teeth |
14th Nov 2016, Monday, 12-1 pm |
| Dr.Vipra GUNETA (SMART) |
Demystifying Adipogenesis: Role of the microenvironment in stem cell differentiation |
7th Nov 2016, Monday, 12-1 pm |
| Dr.Zhu Qingdi (SMART) |
Luminescent and stretchable hydrogel with lanthanide crosslinking |
31st Oct 2016, Monday, 12-1 pm |
| Dr.Liu Mei Jun Jolene (SMART) |
Cartilage regeneration using customized engineering scaffolds |
24th Oct 2016, Monday, 12-1 pm |
| Dr.Ekta MAKHIJA (SMART) |
Nuclear deformability is regulated by cell geometric constraints |
17th Oct 2016, Monday, 12-1 pm |
Dr.Anand Ganesh (SMART) |
Liquid repellent self-cleaning coatings and membranes |
10th Oct 2016, Monday, 12-1 pm |

|
BioSyM Seminar
14 Sep 2016, Wednesday, 4-5 pm
Department of Chemical Engineering,
Texas Tech University, Lubbock, TX
Venue: NUS UTown, Enterprise Level 5, Perseverance Room |
BioSyM Focused Seminar Series
Microdevices in Biological Studies
25 July — 3 October 2016
Speaker |
Title of the Talk |
Date & Time |
Venue |
| Ms. Yasaman NEMAT (NUS / SMART) |
Mechanophenotyping of Breast cancer Cells |
3rd Oct 2016, Monday, 12-1 pm |
CREATE Enterprise Level 5, Perseverance Rooms, UTown |
| Dr.Giulia Adriani (SMART) |
THREE DIMENSIONAL MULTICELLULAR MICROFLUIDIC MODELS |
26th Sept 2016, Monday, 12-1 pm |
| Dr. Bee Luan KHOO (SMART) |
MICROFLUIDIC CLUSTER ASSAY FOR CIRCULATING TUMOR CELLS WITH RELEVANCE IN PATIENT PROGNOSIS |
19th Sept 2016, Monday, 12-1 pm |
| Mr.Joo Chuan YEO (A*STAR, Singapore) |
Tactile Sensing using wearable microfluidics |
5th September 2016, 4-5 pm |
| Dr.Ye Ai (SUTD, Singapore) |
Microfluidic Particle and Cell Manipulation using Surface Acoustic Waves |
29 Aug 2016, Monday, 12-1 pm |
| Dr.Swee Jin TAN (Clearbridge, Singapore) |
Single Cell Applications in Rare Cell Events |
22 Aug 2016, Monday, 4-5 pm |
CREATE Theatrette, Level 2, CREATE Tower @ UTown |
| Professor Patrick Doyle (MIT/SMART) |
Microfluidic Particle Factories |
15 Aug 2016, Monday, 4-5 pm |
CREATE Theatrette, Level 2, CREATE Tower @ UTown |
| Mr. Zhangxing LAI (NUS/SMART) |
Development of Tumour Cell Filtration System for Blood Salvaging Applications |
1 Aug 2016, Monday, 12-1 pm |
CREATE Enterprise Level 5, Perseverance Rooms, UTown |
| Dr.Yin Lu (BioSyM, SMART) |
Applications of Spiral Microchannel Devices in Biomedical Research |
25 July 2016, Monday, 12-1 pm |
 |
| This nine-day “Bootcamp” style course will cover fundamental concepts and practical approaches in understanding cellular functions. The focus is to develop a breadth of knowledge in quantitative techniques to allow bioscience researchers to pursue further depth in their respective research work. Major topics include: gene editing, cellular structure and processes, statistics, bio-imaging, image analysis, modelling, and quantitative methods in biology. There will be extensive tutorials in using Matlab, complemented by hands-on microscopy and wet-lab sessions. Students will work as part of a team to tackle problems and to develop a report on an open area of modern bioscience research. |
BioSyM Focused Seminar Series
Statistics and Optimisations in Biological Studies
13 June — 18 July 2016
Speaker |
Title of the Talk |
Date & Time |
Venue |
| Dr. Sharon Ong (BioSyM, SMART) |
Hidden Markov Models for Microscopy Image Analysis |
18 July 2016, Monday. 12-1 pm |
BioSyM Meeting Room, Enterprise Wing Level 4 |
| Asst. Prof. Timothy Saunders (MBI/NUS) |
the Perils of the P-Value |
27 June 2016, Monday. 4 pm |
CREATE Enterprise Level 5, Perseverance Rooms, UTown |
| Dr. Zhengyun Zhang (SMART) |
Getting the Best of Both Worlds: Diversity in Imaging Measurements |
20 June 2016, Monday. 4 pm |
| Professor H. Harry Asada (MIT / SMART) |
A Data-Driven Approach to Nonlinear System Dynamics, Robotics, and Life Sciences in the Era of Big Data |
13 June 2016, Monday. 4 pm |
CREATE Theatrette, CREATE Tower Level 2 @ UTown,
|
BioSyM Focused Seminar Series
11 April — 6 June 2016
Speaker |
Title of the Talk |
Date & Time |
Venue |
| Prof. Masaaki Kitajima (CENSAM/SMART, Hokkaido University, Japan) |
Investigating Microbial Abundance and Composition in Drinking Water Distribution via Analysis of Biofilm Communities Colonizing In-Pipe Sensors |
16 June 2016, Monday. 4 pm |
CREATE Enterprise Level 5, Perseverance Rooms, UTown
|
| Prof. Yang Liang (SCELSE) |
Chemical Biology Approaches for Biofilm Eradication |
6 June 2016, Monday. 4 pm |
| Dr. Binu Kundukad (SMART) |
Biofilm architecture and composition - determined from the mechanical properties of matrix |
23May 2016, Monday. 4 pm |
| Dr. Thomas Seviour (SCELSE) |
Exopolymers and Biofilm Morphologies |
16 May 2016, Monday. 4 pm |
| Dr. Sreelatha (SMART) |
Bacterial Biofilms and Therapeutic strategies |
9 May 2016, Monday. 4 pm |
| Dr. Durgarao Guttula (SMART) |
|
25 April 2016, Monday. 4 pm |
|
|
18 April 2016, Monday. 4 pm |
|
|
11 April 2016, Monday. 4 pm |
BioSyM Focused Seminar Series
15 Feb — 4 Apr 2016
Speaker |
Title of the Talk |
Date & Time |
Venue |
|
|
4 April 2016, Monday. 4 pm |
CREATE Enterprise Level 5, Perseverance Rooms, UTown |
|
|
21 March 2016, Monday. 4 pm |
|
|
14 March 2016, Monday. 4 pm |
|
|
7 March 2016, Monday. 4 pm |
|
|
29 Feb 2016, Monday, 4 pm |
|
|
22 Feb 2016, Monday, 4 pm |
|
|
15 Feb 2016, Monday, 4 pm |
BioSyM Seminar
Speaker |
Title of the Talk |
Date & Time |
Venue |
|
|
19 Jan 2016, Monday, 4 pm |
CREATE Enterprise Level 5, Perseverance Rooms, UTown
|
|
|
11 Jan 2016, Monday, 4 pm |
on
7 & 8 Jan 2016
Venue: NUS University Hall Auditorium, Level 2, UHall Lee Kong Chian Wing, 21 Lower Kent Ridge Road, Singapore 119077
BioSyM Seminar
Distinguished Visitor Seminar
|
|
Dr. Irina V. Larina
Assistant Professor
Department of Molecular Physiology and Biophysics, Baylor College of Medicine, Houston, Texas, USA
Time: Friday, November 27, 2015, 11 am
Location: CREATE Enterprise Level 5, Perseverance Rooms |
Time: Friday, November 27, 2015, 11.30 am
Location: CREATE Enterprise Level 5, Perseverance Rooms |
BioSyM Seminar
Thematic Seminar Series
on
Fluorescence (Cross-)Correlation Spectroscopy – What do we learn
from fluorescence intensity fluctuations in bio-molecular systems, living cells and embryos?
Every Monday from 16th Nov - 7th Dec 2015
Seminar 2:
Imaging Fluorescence Correlation Spectroscopy
in TIRF and SPIM modes
Dr. Jagadish Sankaran
Research Fellow
Department of Biological Sciences and Centre for Bioimaging Sciences,
National University of Singapore
Time: Monday, November 23, 2015, 4 pm
Location: CREATE Enterprise Level 5, Perseverance Rooms |

Dr. Joby Joseph is Professor in the Department of Physics, IIT Delhi, where he has been a teaching faculty since 1996. He holds Ph.D degree in Physics and M.Tech. degree in Applied Optics from IIT Delhi. He worked as Senior Optical Engineer at Aprilis Inc., Maynard, MA, USA, towards the development of an ultra-high density holographic digital data storage device. He has post-doctoral research experience from Kobe University, Japan and Univ. of Massachusetts, Boston, USA. He is investigator of various research projects from DST, DRDO & DIT. He has supervised many PhD and MTech dissertations, co-inventor in four US patents and has published around 100 research papers in reputed international journals. His current research interests are in Ultra-high density holographic data storage and search methods, 3-D interference lithography for photonic structure fabrication, Digital Holography,
Optical data security, Optical correlators for pattern recognition, Photonic circuits, Biosensors and Photonic Metamaterials.
|
BioSyM Seminar
Prof. Joby Joseph
Photonics Research Lab., Physics Department, IIT Delhi, New Delhi, INDIA.
Light Sculptured Photonic Materials: Fabrication and Applications
Time: Thursday, September 3, 2015, 4 pm
Location: CREATE Enterprise Level 5, Perseverance Rooms
Abstract: Fabrication of photonic structures on relevant length scales can be achieved by means of various techniques such as electron or ion beam writing, deposition methods, self-assembly, interference lithography etc. The prime advantage of interference lithography here is, to fabricate large area defect-free nano-photonic structures both rapidly and cheaply. The presentation gives an overview of the technique of interference lithography in combination with direct laser writing for photonic structure fabrication, and describes novel reconfigurable phase engineering based fabrication technique which is least complex and provide more flexibility compared to existing techniques, at the same time this technique is capable of sculpturing of highly complex photonic structures. Fabrication of complex photonic structures has been achieved with potential for many applications such as, photonic circuits,photonic devices, light extraction, optical tweezers, biosensors, metamaterials etc.
|
BioSyM Seminar
Ms Tan Ying Ying
Senior Product Manager, Fisher Scientific
Proper Pipetting and Pipetting Ergonomics
Time: Monday, August 24, 2015, 4 pm
Location: CREATE Enterprise Level 5, Perseverance Rooms
|
BioSyM Seminar
Dr. Chen Wen,
Department of Electrical and Computer Engineering, NUS
Optoelectronic imaging and its applications
Time: Monday, August 17, 2015, 4 pm
Location: CREATE Enterprise Level 5, Perseverance Rooms
|
 |
This 2-week “Bootcamp” style course will introduce researchers from biology or physical science backgrounds to key fundamental concepts and practical approaches in understanding cellular functions. The focus is to develop a breadth of knowledge that allows bioscience researchers to pursue further depth in their respective research work. Major topics include the Central Dogma of Molecular Biology, gene cloning and editing, microscopy and bioimaging, image analysis
and quantitative methods in biology and the choice and limitations of model organisms.
The Bootcamp lectures are open to all interested graduate students and post-docs. For graduate students, the lectures will be reinforced in thematic-based practicals, leading to 2-MC.
Where:
CREATE Theatrette, Level 2, CREATE TOWER, U-Town
Registration and Info: www.mbi.nus.edu.sg/events/2015bootcamp |

|
SMART Biosym Seminar by
Prof. Yan Jie (NUS - Physics & Mechanobiology Institute)
Time: Monday, July 13, 2015, 4 pm
Location: CREATE Enterprise Level 5, Perseverance Rooms |

Dr. Kalpesh is currently a postdoctoral associate in BioSystems and Micromechanics Inter-Disciplinary Research Group of Singapore-MIT Alliance for Research and Technology (SMART). He did his masters from Indian Institute of Technology, Kanpur in Electrical Engineering. He received his Ph.D. in Optical imaging area, from the NUS Graduate school of integrative science and engineering (NGS). After his PhD he worked as an associate scientist at cognitive science program, Temasek Laboratories. |
SMART Biosym Seminar by
Dr. Kalpesh Mehta (SMART- BioSyM)
Spread spectrum method based diffuse optical tomography for brain imaging
Time: Monday, July 6, 2015, 4 pm
Location: Enterprise Level 5, Perseverance Rooms
Extraction of functional information such as bimolecular information are important in biological applications, as they are the precursor to the structural changes in tissues. In my talk I will discuss about novel diffuse optical imaging system as a modality to extract the hemodynamic information.
Conventional diffuse optical imaging system uses pulse laser as a source. Use of pulse laser makes system design bulky and expensive. In our work we have proposed novel spread spectrum method based diffuse optical tomography system. The proposed system is fast, compact and accurate. The proposed system has been used for the brain imaging applications to monitor the cognitive state. |

Balpreet Ahluwalia is Associate Professor at the Department of Physics & Technology, University of Tromsø, The Artic University of Norway. He did his PhD from Nanyang Technological University, Singapore in 2007. His research interests include development of optical nanoscopy/ optical tweezers and application of optical nanoscopy for relevant biological problems. Dr Ahluwalia has published over 50 journals and conference papers. Ahluwalia is recipient of European Union prestigious ERC Grant on “Integrated Nanoscopy” in 2013, the grant supports 2 Million € for the next 5 years. In addition Ahluwalia is also a recipient of UiT-Strategic Funding (Award of 1 Million €). Ahluwalia has worked in different research labs in USA, UK, Norway and Singapore during his young research career. |
SMART Biosym Seminar by
Dr. Balpreet Ahluwaliya
Associate Professor, University of Trosmo, Norway
Optical Nanoscopy of living cell to decode nanoscale biological system
Time: Monday, July 2, 2015, 11 am
Location: Enterprise Level 5, Perseverance Rooms
Optical nanoscopy allows to study biological and functional processes of sub-cellular organelles. In structured illumination microscopy (SIM) the sample is illuminated with a grid-like interference pattern to encode higher spatial frequency information into observable Moiré patterns. By acquiring multiple images and a computation trick a super-resolved image is obtained. While in single molecule localization, single emitter are localised with 30-50 nm resolution to provide enhancement in the resolution. In this talk, I will discuss the opportunities and challenges of imaging living cells using SIM and imaging of fixed cells using dSTORM. Different applications of optical nanoscopy of living/fixed cells will be discussed; a) imaging of mitochondria in a keratinocyte cell and b) cell-to-cell HiV viral transfection, c) Imaging of membrane nano-holes (Fenestration) in liver endothelium cells.
|

Dr. Yin is currently a postdoctoral associate in BioSyM-SMART. He received his Ph.D. in Computational Systems Biology, from SMA Programme, NUS. He was a research fellow and then postdoctoral associate in Infectious Disease Inter-Disciplinary Research Group of SMART from 2010 to 2012, when he used imaging-based tissue informatics approach to explore the cellular mechanism of lung tissue repair following influenza infection in mouse models. His current research focuses on the development and application of inertial microfluidics in various stem cell and cancer studies, including circulation tumour cell, tumour stem cell, adult lung stem cell, cartilage and spinal chondrocytes, etc.
|
SMART Biosym Seminar by
Dr. Lu Yin (SMART- BioSyM)
Microfluidic-assisted isolation of club cells with notable traces of stemness from the mouse lung
Time: Monday, June 29, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
Club cells are non-ciliated bronchiolar epithelial cells that line up in the interior wall of bronchioles. Previous studies have reported the function of club cells in protecting bronchiole lining, detoxifying harmful substances getting into the airway through inhalation, and repairing bronchiolar injury by renewing itself and differentiating into ciliated cells. More recent studies have demonstrated in vivo that in the case of severe lung injury, club cells are able to differentiate into alveolar epithelial cells and P63-expressing cells to repair the damaged alveoli and regenerate new bronchioles in damaged lung parenchyma. The emerging understanding of club as multi-potent progenitor/stem cell has shed light on the potential stem cell therapy approaches in clinical management of severe lung tissue damage, and this requires isolation of high potency club cells. In this study, we established a novel method for the isolation of multi-potent club cell from mouse lung using a combination of inertial microfluidics and flow cytometry sorting. Our method has significantly improved the efficiency and purity of club cell isolation, and was able to enrich thepopulation of club cell with stronger colony forming capacity.
|
Thursday, 25 June 2015
@ CREATE Theatre, CREATE Tower-Level 2, 1 Create Way
Singapore 138602
Organized by BioSyM, Singapore-MIT Alliance for Research and Technology (SMART), the workshop on metastatic cancer aims to give an overview of the ongoing collaborative research and to also seek new collaboration opportunities. The talks will be centered on the recent research with a focus on future challenges. Registration is open to all interested researchers and students. Please RSVP bala@smart.mit.edu by 22nd June 2015 (Monday).
SMART Biosym Seminar by
Mr. Evan Tan (NUS / SMART- BioSyM)
Role of JAG1-extracellular vesicles on angiogenesis
Time: Monday, June 22, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
Extracellular vesicles (EVs) are novel mediators of cellular signaling. They are able to ferry growth factors, receptors, nucleic acids and lipid mediators. Studies involving tumor-derived EVs have shown that they have been able to promote tumor progression. They are able to promote angiogenesis, tumor proliferation and metastasis. Jagged1(JAG1), a key notch ligand involved in angiogenesis, has been shown to be up-regulated in several metastatic cancers. In breast cancers, tumor-derived JAG1 promotes bone metastasis. High levels of JAG1 and Notch1 co-expression in breast cancer patients is associated with poorer overall survival. In prostate cancer, down-regulation of Notch1 and JAG1 resulted in an inhibition of cell growth, migration and invasion while promoting cell death. Hence, tumor-derived JAG1-EVs could potentially be a novel mediator of tumor progression by regulating angiogenesis.
|

Shakil is a Research Scientist at SMART and has joint appointment at the two IRG’s: CENSAM and BIOSYM. He did his PhD in Physics from the University of Sydney,
Australia and has previously worked at AIST, Tsukuba, Japan and University of
Colorado at Boulder, USA. In Singapore, he has worked for the Department of
Biomedical Engineering at NUS and at SERI (Singapore Eye Research Institute).
His research interests are in optical imaging, imaging through turbid media, phase
imaging, digital holography, confocal and multi-photon microscopy. Shakil applies his optical imaging and analysis expertise in problems, such as visualization of cells and cellular structure in thick tissue samples and improving the
optical imaging contrast in turbid media like, a biological tissue and particulate imaging and characterization in highly turbid water, and 3D optical visualization.
|
SMART Biosym Seminar by
Dr. Shakil Rehman (SMART- BioSyM & CENSAM)
Time: Monday, June 15, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
Human visual perception is based on the interpretation of visual motion: a process by which the description of environment in terms of objects, their 3D shape and their motion through space is constructed on the basis of the changing image that reaches the eye. Here I will use multiple view geometry: a method for implementing a multiple view stereo system to reconstruct 3D shapes of the real world objects from a set of 2D images.
|
SMART Biosym Seminar by
Ms. Sonia Brady
Vanderbilt University, USA
Time: Monday, June 8, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
We focus on using single molecule techniques such as optical trapping and single molecule fluorescence spectroscopy to investigate cellular and molecular machinery such as kinesins, ClpXP, amyloid fibers, and peptide recognition through the TCR-pMHC interaction. The talk will specifically focus on our work with cellobiohydrolase 1 from Trichoderma reesei, which processively hydrolizes cellulose into cellobiose units. We developed a single molecule optical trapping assay to track TrCel7a with nanometer
resolution in which we discover steps of approximately 1nm, a single cellobiose unit. In addition to investigating the effect of force and temperature on this motor, we probed isolated TrCel7a domains to gain insight into a motility mechanism and determine that the catalytic domain alone is sufficient for processive motion.
|

Mr. Tengyang Jing is currently a PhD student and recipient of SMART fellowship at the National University of Singapore (NUS). He has received his B.Eng. degree in Electrical Engineering in 2011 from NUS. His research interests lie in microfluidics for cell assay, especially for single-cell analysis.
|
SMART Biosym Seminar by
Mr. Tengyang Jing, NUS / SMART- BioSyM
Droplet-based single cancer cell protease study
Time: Monday, May 25, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
Single-cell assay becomes increasingly attractive because it reveals the function and behavior of individual cells in a population of heterogeneous cells. It enables the investigation of rare cell subgroup, whose cell signals are usually masked by average response from cell population. Here, we intend to study protease secretion activity of single viable tumor cells using droplet microfluidic platform. The functional assay is achieved by encapsulating cancer cells into picoliter droplets in oil environment together with fluorescent substrate. Protease secreted from cancer cells cleaves the polypeptide target of the substrate and releases fluorophores that bright up cell-containing droplets. This droplet-based platform helps us understand various protease secretion activities of different cancer cell lines and can be potentially applied on the study of patient-derived cell sample for better diagnosis and prognosis.
|

Dr. Zhang is currently a Research Scientist in BioSyM Inter-Disciplinary Research Group of Singapore-MIT Alliance for Research and Technology (SMART). He received his Ph.D. in condensed matter physics, from Institute of Physics, Chinese Academy of Sciences. He was a patent examiner at State Intellectual Property Office of China from 2009 to 2010. His current research interests are diversity of DNA structures, and initiation of biofilm formation.
|
SMART Biosym Seminar by
Dr. Xinghua Zhang, SMART- BioSyM
Diversity of DNA structures under high tension
Time: Friday, May 15, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
DNA has multiple structures depending on conditions. Previous single-molecule studies revealed that mechanical stretching of force could induce formation of overstretched DNA structures from the most common B-form DNA structure. Three possible structures have been proposed and debated for 17 years, namely: 1) peeled ssDNA, 2) DNA bubble, and 3) S-DNA. By thermodynamics, mechanical and electrostatic studies, we have successfully identified all the three proposed structures. We also found interconversions between overstretched DNA structures can be induced by changing buffer conditions.
The published papers were highlighted by Biopolymer on Volume 97(8), released twice by NUS Science Press Releases, and reported by dozens of medias. Currently, we are studying the physiological functions of the three overstretched DNA structures, and explore the presence of any new DNA structures under both force and torque. |
|
SMART Biosym Seminar by
Dr. Shih‐Chi Chen
Assistant Professor, Department of Mechanical and Automation Engineering,
The Chinese University of Hong Kong
Time: Tuesday, May 14, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms,
1 Create Way, Singapore 138602 |
SMART Biosym Seminar by
Dushan Nawoda Wadduwage
Graduate student, SMART- BioSyM
New Common path interferometer design for high-throughput wide-field spectral imaging
Time: Monday, May 11, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms |
Imaging Fourier transform spectroscopy (IFTS) is a powerful method for resolving complex spectra especially from unknown endogenous molecules. IFTS can be operated in wide-field mode allowing its use with many high throughput, high content image cytometers. There are a number of limitations in existing wide field IFTS designs. Non-common path designs are often phase unstable. Alternatively, designs based on common-path Sagnac interferometer are very stable and are commonly used. However Sagnac designs can only be operated in exhaustive sequential acquisition modes and hence are incompatible with high throughput imaging.
In this talk we present a novel phase stable common-path interferometer with the aim for full-field IFTS that allows strategic data acquisition. Our simulations suggest that the instrument is order of magnitudes faster for wide-field spectral measurements. |

Sebastien Uzel is a graduate student at MIT in the department of Mechanical Engineering and a research assistant in the Kamm and the So lab. He is currently a visitor at BioSyM as part a the Singapore-MIT Alliance for Research and Technology (SMART) program. He received a Master’s degree in Mechanical Engineering from Ecole Centrale Paris, France and in Biomechanics from Ecole Nationale Superieure d'Arts et Metiers (now Arts et Metier, ParisTech), Paris, France. His current research is focus on microfluidic and optogenetic as a way to enhance the physiological relevance and controllability of in vitro neuromuscular tissues.
|
SMART Biosym Seminar by
Sebastien Uzel
Graduate student, MIT / SMART- BioSyM
Microfluidic and Optogenetic Technologies to Model Neuromuscular Junction Formation and Function
Time: Monday, May 4, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
Neuromuscular junctions (NMJ) have always been the subject of extensive research, either as a universal synapse model, or in order to overcome the challenges posed by neurodegenerative disorders or nerve injuries. In this project, we describe a new microfluidic design that can host a three-dimensional (3D) and compartmentalized co-culture of skeletal muscle and motor neurons. We demonstrated the ability for the neurons to extend their neurites within the extracellular matrix toward a functional muscle bundle and form NMJs, while allowing for passive and non-invasive measurement of the force generated by the muscle tissue. In order to facilitate the excitation of the motor neurons and interrogate the NMJs, we targeted mouse embryonic stem cells (ESC) with Channelrhodopsin, a light sensitive microbial ion channel, and created a stable cell line, serving as a pool for photosensitive motor neurons. After demonstrating the first formation of light activate NMJ in vitro, we show that these neurons can be used to remotely triggered muscle contraction in our microfluidic device upon optical stimulation. |

Vijay Raj Singh received Master of Technology (M.Tech.) degree in Applied Optics from IIT Delhi, India in 2003, and Ph.D. degree in Optical Engineering from NTU, Singapore in 2008. From 2007-2010, he worked at AEM Singapore Pte Ltd and NTU, Singapore and focused on development of digital holographic microscope tool as table-top and handheld systems for dynamic mechanical characterization of microelectronic devices. Currently he is working with SMART centre, Singapore and his research interests include 3D optical microscopy methods for biomedical imaging applications, quantitative phase imaging methods, image reconstruction and image processing algorithms development.
|
SMART Biosym Seminar by
Dr. Vijay Raj Singh
Research Scientist, SMART - BioSyM
Confocal reflectance quantitative phase microscope for studying cell and nuclear membranes mechanics
Time: Monday, April 27, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
In order to generate the contrast in unstained cell, phase resolved techniques are available. Over the past two decades, significant advancement of quantitative phase microscopy (QPM) have been made, which allow measurement of cellular plasma membrane fluctuation with resolutions on the scale of nanometres and milliseconds, and used for staging of malaria parasite infection of red blood cells (RBCs). The developed QPM methods are mainly transmission based and have good sensitivity and speed but these methods lack three-dimensional (3D) resolution. Studies show that changes in nuclear mechanics are accompanied by defects in gene expression and a distorted nuclear morphology. Most recently, it is shown that the nuclear membrane deformation is suggested as a marker for isolating mesenchymal stem cells (MSCs) from bone marrow. Among the several other biophysical markers considered for MSCs, it was found that cell stiffness and nuclear membrane fluctuations correlated strongly with multipotency. In this talk a newly developed confocal reflectance quantitative phase microscopy system is presented, that can be used to study the biomechanics of intracellular level structures. This reflection phase microscope will enable non-invasive, marker-free, 3D dynamic imaging of dimensional fluctuations of both the cellular plasma and nuclei membranes. |
|
SMART Biosym Seminar by
Dr. Sergey Y. Tetin
Diagnostics Research, Abbott Diagnostics Division
Time: Tuesday, April 21, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms,
1 Create Way, Singapore 138602 |
|
SMART Biosym Seminar by
Prof. Chris Kaiser
Professor of Biology, MIT
Time: Monday, April 20, 2015, 4 pm
Location: CREATE Theatrette, CREATE Tower (Level 2)
1 Create Way, Singapore 138602 |

Poon Zhiyong is a research scientist at BioSyM who is broadly interested in the development of cell- and nanoparticle-based treatments for human disorders.
|
SMART Biosym Seminar by
Dr. Poon Zhiyong
Research Scientist, SMART - BioSyM
Clinical use of mesenchymal stem cells for human therapy in Singapore
Time: Monday, April 13, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
Mesenchymal stem cells can be extracted from human bone marrow, expanded in vitro and subsequently re-implanted back into patients for the treatment of several hematopoietic, orthopedic and inflammatory disorders. The efficacies of such treatment have however been variable across different trial centers and this remains an important challenge to their more widespread use. I will discuss how biophysical cell characterization approach can help improve the consistency of this therapy, with emphasis on the clinical trials that are being conducted in Singapore. |

Chen‐Yuan Dong received his PhD in physics from the University of Illinois at Urbana‐Champaign 1998 and went to MIT’s Mechanical Engineering Department as an NIH postdoctoral fellow. In 2001, Dr. Dong joined the Department of Physics at National Taiwan University where he is now a Distinguished Professor. He specializes in the development and applications of advanced optical microscopy techniques for elucidating fundamental processes in biology and potential applications in medicine. He has over 100 SCI publications with a number of his works selected as journal issue covers. He is a Fellow of the Optical Society of American and serves on the editorial board of the Journal of Biomedical Optics.
|
SMART Biosym Seminar by
Dr. Chen‐Yuan Dong
Department of Physics
National Taiwan University
Development and Applications of Multiphoton
Microscopy in Biomedical Research
Time: Thursday, April 9, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
Traditionally, the difficulty of accessing internal organs has often limited the study of complex cellular physiology to in vitro, monolayer systems. While in vitro studies using techniques such as optical imaging and electron microscopy have provided a wealth of dynamic and structural information of cells and tissues, the critical components of physiological phenomena in complex three‐dimensional tissues are missing. Therefore, much of our efforts have been focused on developing advanced multiphoton microscopy techniques, leading to the study of physiological phenomena in vivo. In the process, we found that multiphoton imaging can be useful in medical diagnostic, evaluation of tissue engineering products, and the elucidation of dynamic processes in vivo. Future developments and impacts of our approach in biomedical research will be discussed. |
SMART Biosym Seminar by
Dr. Sreelatha Sarangapani (SMART)
Short distance probes for the conformational dynamics with tmFRET
Time: Monday, April 6, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
Abstract: Protein conformational space is essential to understand the function. tmFRET can be used, to determine distances from multiple locations within a protein at extremely low concentrations.
Fluorescence resonance energy transfer (FRET) between a small fluorescent dye and transition metal ions have been suggested to monitor small structural rearrangements in proteins. Here, we engineered a spectral series of optimized transition metal ion-binding protein that respond to metals with large changes in fluorescence intensity. The engineered proteins can act as metal biosensors or imaging probes whose fluorescence can be tuned by metals. This improved method overcomes several of the key limitations of classical FRET for intramolecular distance measurements.

Timothy Lu, M.D., Ph.D. is an Associate Professor leading the Synthetic Biology Group in the Department of Electrical Engineering and Computer Science and the Department of Biological Engineering at MIT. He is a core member of the MIT Synthetic Biology Center and a co-founder of Sample6 Inc., Synlogic Inc, and Eligo Biosciences.
Tim’s research at MIT focuses on engineering computing and memory circuits in living cells, applying synthetic biology to tackle important medical and industrial problems, and building living biomaterials that integrate biotic and abiotic functionalities. He is a recipient of the NIH New Innovator Award, the Presidential Early Career Award for Scientists and Engineers, and the Ellison Medical Foundation New Scholar in Aging Award, among others.
|
SMART Biosym Seminar by
Professor Timothy Lu
MIT
Translating Synthetic Biology to Human Health Applications
Time: Thursday, March 26, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
Synthetic biology is an emerging discipline for the accelerated and increasingly precise engineering of biological systems to achieve novel functions. Most applications for synthetic biology to-date have focused on the metabolic engineering of prokaryotes or simple eukaryotes to manufacture small-molecule products. However, in recent times, platform technologies for engineering sophisticated genetic circuits in living cells have advanced considerably. The technologies promise to enable the programming of precise and dynamic sense-and-respond behaviors in bacteria and human cells. Furthermore, the remarkable success and progression of cell and gene therapies in human clinical trials is opening the door for more sophisticated therapeutics for human diseases.
Due to technological convergence, regulatory progress, and market interest, there is an exciting opportunity for Translational Synthetic Biology to address important human health applications that are not readily tackled with current approaches. For example, next-generation cell therapies would benefit from regulated gene circuits that enable safety switches, specific targeting, integrated sense-and-respond behaviors, and powerful effector functions. Next-generation gene therapies could dynamically modulate multiple genes in cells to achieve enhanced therapeutic efficacy over single gene approaches. Furthermore, accurate, rapid, and systems-level diagnostics will need to be coupled with personalized therapeutics to achieve the potential benefits of precision medicine.
I will discuss our work in foundational synthetic biology, including the development of integrated logic-and-memory systems, analog computing, and dynamic genome editing in living cells. Furthermore, I will describe our efforts to translate synthetic-biology technologies to real-world applications via two venture-backed startups working to create rapid microbial diagnostics and microbiome therapeutics. Finally, I will present our thoughts on areas of research in next-generation cell and gene therapies that may benefit from synthetic biology. |

After finishing his MSc studies at Leiden University, Hans Heemskerk did a PhD on exon skipping as a therapy for Duchenne Muscular Dystrophy (DMD) at the same university. After receiving his PhD he was a postdoctoral researcher at the Institute of Child Health of UCL in London, where he worked on stem cells as a potential therapy for DMD. Research on potential therapies is focussed on the end result and because Hans is also interested in the details of the processes that take place inside the body, he came to SMART in 2013 as a SMART scholar to perform detailed in vivo and ex vivo imaging of skeletal muscle regeneration.
|
SMART Biosym Seminar by
Dr. Johannes Antonius Heemskerk
SMART
Detailed imaging of muscle regeneration
Time: Monday, March 23, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
To a larger or smaller extent, ineffective muscle regeneration is involved in diseases and conditions like Duchenne Muscular Dystrophy, pressure ulcers and sarcopenia. Muscle regeneration is a complicated process involving inflammation, fibrosis and the formation of new muscle fibers by satellite cells, the local stem cell in the muscle. Although a lot is known about muscle regeneration it has never been followed over time in vivo, and therefore a lot of details are still unclear. More detailed knowledge of what happens inside the body can help us to better predict and evaluate the effectiveness of potential therapies. And it might even lead to the discovery of new targets for therapies. In this talk, I will present the animal model we are using to find these details and the results of the intramuscular experiments.
|

Sharon received her doctoral degree in Mechatronics Engineering from the University of Sydney, Australia in 2008. Currently, she is a Research Scientist at the Singapore-MIT Alliance for Research and Technology (SMART) with Harry Asada. Her research interests include computer vision, pattern recognition and stochastic modelling.
|
SMART Biosym Seminar by
Dr. Sharon Ong
SMART
Towards Reliable Segmentation and Tracking of Cell Population Morphologies from Microscopy Images
Time: Monday, March 16, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
In microscopy image processing, it can be hard to determine cell and nuclei boundaries as there is image noise and cells may be clustered together or overlapping. When we extract cells manually, we apply some prior knowledge of what its nuclei and cell should look like. This talk will discuss an approach to jointly estimate shapes and poses of stained nuclei from confocal microscopy images and cells from time-lapse phase contrast images using statistical prior information. |

|
SMART Biosym Seminar by
Dr. Krishna Agarwal
SMART
Time: Monday, March 9, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms |
|
SMART Biosym Seminar by
Dr. Liu Xiaogang
SMART
Time: Thursday, March 5, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
Structure-Property Relationships for Developing Near-Infra-Red Fluorophores
Organic fluorophores (dyes) have been used extensively as bio-molecular labels, chemical sensors and cellular stains for chemical biology research and medical diagnosis. Recently, one conspicuous area for further research is the design and synthesis of dyes that emit further into the near-IR region for deep tissue penetration. In this talk, I will firstly examine the structure-property relationships of fluorophores at the molecular level, and then demonstrate the utilizations of these relationships in developing new classes of novel near-infra-red dyes. The molecular aggregation effects of dyes when dissolving in solution will also be addressed. |

Taishi is a PhD student with Prof Matthew Lang and Qing-Hua Xu at National University of Singapore. He obtained his B.Eng degree (1st class honours) from Nanyang Technological University. His research focuses on nanophotonics and their applications in fluorescence enhancement.
|
SMART Biosym Seminar by
Mr. Zhang Taishi
NUS / SMART
Gold Nanoparticles as Optical Antennas for Single-molecule Fluorescence Enhancement
Time: Monday March 2, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
Abstract:
Gold nanoparticles (NP) permit efficient energy transfer between far-field propagating light and near-field localized surface plasmon resonance (LSPR), and thus function as an antenna in optical frequency. This property has been utilized in the enhancement of optical responses such as Raman scattering and fluorescence. In this talk I will present our recent work using DNA origami as a nano breadboard to fix the NP dimers in close proximity with aligned pattern, their plasmon coupling enables even higher fluorescence enhancement compared with single NP. The near-field fluorescence enhancement is sensitive to the distance between NP and fluorophore, and is investigated for a single-molecule sensor for molecular conformation change. |
|
Department of Biological Science, NUS, Biology Colloquium
Prof.Peter So
MIT / SMART
Time: Monday, February 27, 2015, 4 pm
Location: DBS Conference Room, DBS, NUS |
SMART Biosym Seminar by
Dr.Binu KUNDUKAD
SMART
Time: Monday February 16, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms

Zhiyang's research designs biologically relevant features for image analysis, builds computational models to simulate dynamic morphological change, and adapts methods of statistical machine learning for interpretation of spatiotemporal imaging data.
|
SMART Biosym Seminar by
Dr. Tam Zhi Yang
SMART
Mitochondrial dynamics and quality control in ageing
Time: Monday February 09, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
Abstract: Mitochondria are important organelles in eukaryotic cells. The decline in mitochondrial function has been postulated as one of the causes of ageing. Aged cells in post mitotic tissues, such as heart, brain and skeletal muscles, frequently have deficient respiratory capacity due to mitochondrial dysfunction. Incidentally, these cells are often associated with an accumulation of mtDNA mutations to high burden, exceeding a certain threshold necessary for sustaining mitochondrial function. Intuitive understanding of clonal expansion of mutant mtDNA is impeded by the complexity of mitochondrial dynamics and intracellular mtDNA organization. In this talk I will be presenting insights into the role of mitochondrial fusion-fission using in silico and in vivo models.
|
|
SMART Biosym Seminar by
Dr. Dai Liang
SMART
Time: Monday February 02, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
|
|
SMART Biosym Seminar by
Time: January 29, 2015, 4 pm
Location: SMART Enterprise Level 5, Perseverance Rooms |
SMART Biosym Mini-Workshop on
Time: January 30, 2015, 10.30 am - 12 noon
Location: SMART Enterprise Level 5, Perseverance Rooms
|
|
SMART Biosym Seminar by
Time: Monday January 19, 2015, 5 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
|
|
SMART Biosym Seminar by
Time: Wednesday January 14, 2015, 5 pm
Location: SMART Enterprise Level 5, Perseverance Rooms
|
|
SMART Biosym Seminar by
Time: Tuesday January 13, 2015, 11:00‐12:00
Location: SMART Enterprise Level 5, Perseverance Rooms
Contact: Peter So, ptso@mit.edu |
12th Jan 2015
@ Perseverance Rooms, Level 5, Enterprise Wing, CREATE
|
Venue: CREATE Theatre, CREATE TOWER, Level 2
Date: 9th Jan 2015
Time: 2.30 pm |
@ CREATE Theatre (CREATE Tower Level 2) on 8 & 9 January 2015
BioSyM Friday Seminar by Dr. Sebastian Beyer
Date: 28th November, 2014, 4.00 pm @ Enterprise Level 5 Perseverance Rooms, CREATE
Title: "Carbohydrate colloids for biomedical applications”
BioSyM Friday Seminar by Dr. Guan Zhenping
Date: 21st November, 2014, 4.00 pm @ Enterprise Level 5 Perseverance Rooms, CREATE
Title: "Enhanced NIR absorption in coupled Au NSs and its application in E coli photothermal imaging”
BioSyM Friday Seminar by Dr. Darwin Tay
Date: 14th November, 2014, 4.00 pm @ Enterprise Level 5 Perseverance Rooms, CREATE
Title: "A Biological Continuum based Approach for Efficient Clinical Classification”
BioSyM Friday Seminar by Dr. Zhang Zhengyun
Date: 7th November, 2014, 4.00 pm @ Enterprise Level 5 Perseverance Rooms, CREATE
Title: "Phase Imaging using shifted wavefront sensor images”
BioSyM Friday Seminar by Dr. Krishna Agarwal
Date: 31st October, 2014, 4.00 pm @ Enterprise Level 5 Perseverance Rooms, CREATE
Title: "Fluorescence intermittence and imaging”
BioSyM Friday Seminar by Mr. Evan Tan
Date: 24th October, 2014, 4.00 pm @ Enterprise Level 5 Perseverance Rooms, CREATE
Title: "The role of JAG1 exosomes in angiogenesis”
BioSyM Friday Seminar by Ms. Wang Mengmeng
Date: 17th October, 2014, 4.00 pm @ Enterprise Level 5 Perseverance Rooms, CREATE
Title: "Automated Tracking of Cells Embedded in Angiogenic Sprouts from Phase Contrast Images with Multiple Hypothesis Kalman Filters”
BioSyM Friday Seminar by Dr. Johannes Antonius HEEMSKERK
Date: 10th October, 2014, 4.00 pm @ Enterprise Level 5 Perseverance Rooms, CREATE
Title:"Imaging of muscle regeneration in the confetti mouse model"
BioSyM Friday Seminar by Ms.Dulani
Date: 3rd October, 2014, 4.00 pm @ Enterprise Level 5 Perseverance Rooms, CREATE
Title: ''Targeted Immunomodulation Therapy Using Folic-Acid-CCL21-coated Nanoconjugates''
BioSyM Friday Seminar by Mr.Ho Yan Teck
Date: September 26, 2014, 4.00 pm @ Enterprise Level 5 Perseverance Rooms, CREATE
Title: "Nanoparticle rheology in circulation"
BioSyM Friday Seminar by Dr.Peng Weng Kung
Date: September 19, 2014, 3.30 pm @ Enterprise Level 5 Perseverance Rooms, CREATE
Title: "System biology: How true is the ambient glucose in diabetic patient reflecting the risk prediction of secondary complication?"
BioSyM Friday Seminar by Dr.Vijay Raj Singh
Date: September 12, 2014, 4 pm @ Enterprise Level 5 Perseverance Rooms, CREATE
Title: Holography based methods for 3D bio imaging applications
BioSyM Friday Seminar by Ms. Yasaman
Date: August 22, 2014, 4 pm @ Enterprise Level 5 Perseverance Rooms, CREATE
BioSyM Friday Seminar by Mr. Jing Tengyang
Date: August 15, 2014, 4 pm @ Enterprise Level 5 Perseverance Rooms, CREATE
Title: Microfluidic device for single circulating tumor cell (CTC) analysis
BioSyM Friday Seminar by UnderGraduate Students
Date: July 25, 2014, 4 pm @ Enterprise Level 5 Perseverance Rooms, CREATE
Special seminar by Prof. Bradley Olsen from Chemical Engineering, IT, who has designed an impressive class of protein-polymer gels.
Date: 24 July 2014, 4pm @ Enterprise Level 5 Perseverance Rooms.
BioSyM Friday Seminar by Dr.Majid Ebrahimi Warkiani
Date: July 18, 2014, 2.30 pm @ Enterprise Level 5 Perseverance Rooms, CREATE
Title: High Throughput Microfluidic Cell Sorting Technologies and Applications
Instructors and Organizers:
Dr. Sharon Ong, Research Scientist at BioSyM & Professor Harry Asada, BioSyM PI and Ford Professor of Engineering, MIT
When & Where:
June 9th 2014 to June 20th 2014 on Mondays,Wednesdays and Fridays from 10:00 a.m. to 12:00 noon, at Level 5, Enterprise Wing.
Past Events
BioSyM Seminar Series 2014
Prof. Yong-Ak (Rafael) Song, Assistant Professor, New York University Abu Dhabi will present "New Microchip Technologies for Manipulation of Biomolecules and Ions in Bioengineering".
Date: 25th April 2014, Time: 3.30 pm, Venue: BioSyM, SMART Centre,
1 CREATE Way, Level 4, Enterprise Wing, Singapore 138602
Biomedical Imaging Seminar on April 4, 2014: 2-4 pm @ Perseverance Room, Level 5, Enterprise Wing, CREATE, UTown
Speakers:
1. Professor Karsten König, JenLab GmbH, Schillerstr. 1, 07745 Jena, Germany, www.jenlab.de
& Department of Biophotonics and Laser Technology, Saarland University, Campus A5.1, 66123 Saarbrücken, Germany
"In vivo Optical Biopsies using Multiphoton/CARS Tomographs"
2. Dr. Aisada Uchugonova, Saarland University, Department of Biophotonics and Laser Technology,
Campus A5.1, 66123 Saarbruecken, Germany
"Femtosecond Laser Microscopy of Stem Cells"
NUS Mechanical Engineering Seminar by Prof.Harry Asada (BioSyM PI)
"Wearable Robots for Manufacturing and Health Care"
Abstract: Imagine that one day, you have a third arm and a third leg attached to your body. The extra limbs will help you hold objects, support your body, share a workload, and streamline the execution of a task. If the movements of such Supernumerary Robotic Limbs (SRL) are tightly coordinated with your own arms, you may come to perceive the extra limbs as an extension of your body, incorporated into your body image. The objective of this work is to develop key enabling technologies for transforming robots to act as parts of a human body. Wearable SRLs are opening up new horizons of robotics, posing diverse research issues and challenges ranging from machine design and human-robot coordination, to biomechanics, motor control, and machine learning and perception.
Three types of Supernumerary Robotic Limbs being built at the d’Arbeloff Lab of MIT will be presented: 1) a lightweight robot sitting on the shoulder of a human for lifting and supporting objects in the overhead area, 2) a seven-fingered hand (5 fingers + 2 robotic fingers) for grasping and manipulating large/odd-shaped objects, and 3) a pair of wearable canes attached around the waist for supporting and bracing the human body. For these wearable robots, communication and coordination with the human is the key challenge. Three aspects of coordination control will be presented. First, the concept of biological synergies is applied to the seven-fingered hand in order to control the two robotic fingers in concert with the five human fingers. Through grasp experiments and data analysis using Principal Component Analysis it will be shown that synergies
exist for seven-fingered hands as well as for five-fingered hands. For real-time control, Partial Least Squares (PLS) regression is used for extracting control laws from the data that can best correlate the posture of the two robotic fingers to that of the five human fingers. Second, interactive human-robot-task processes are modeled as a concurrent, distributed event system based on Coloured Petri Net (CPN). A type of hybrid control system is constructed by replacing CPN’s static state transitions with dynamic, proactive transition laws learned from human demonstration data. Finally, a learning algorithm inspired by biological muscle training is applied to the wearable canes. Untrained robotic actuators are treated as un-innervated muscles. Repeated exposures to simultaneous physical and informational stimulations lead to formation of artificial neuromuscular junctions that control the wearable robots. The seminar will be concluded with potentials of wearable SRLs and their social and economic impacts, ranging from increasing productivity and safety for factory, construction, and field workers to improved quality of life for elderly people and the handicapped as well as reduced workload for caregivers and clinicians.
Date: 14th Jan 2014 @ 12.30 to 1.30 pm
Venue: CREATE Theatre (CREATE Tower Level 2) on the CREATE/UTown campus of the National University of Singapore (NUS)
BioSyM Annual Workshop and Scientific Advisory Board (SAB) Meeting
Dates: Jan 13 & 14, 2014
Venue: CREATE Theatre (CREATE Tower Level 2) on the CREATE/UTown campus of the National University of Singapore (NUS)
BioSyM Seminar by Prof.Viola Vogel of ETH, Zurich, Switzerland
Topic: Nanomechanics by which cells explore their environments
Abstract: Investigating the mechanisms how cells explore their extracellular environments and catch their prey is not only scientifically exciting but will also lead to new technological applications. Cells recognize physical features in their environments by exploiting mechanical forces generated by their motors which pull on distal extracellular anchoring points. Major advances in this field have been made possible by novel micro- and nanofabrication techniques to engineer precisely controlled cell environments, combined with novel nanoanalytical techniques to probe the mechanics of single molecules, cells and tissues, as well as by advanced imaging techniques.
Date & Time: 10 Jan 2014 @ 10.30 am
Venue: CREATE Theatre (CREATE Tower Level 2) on the CREATE/UTown campus of the National University of Singapore (NUS)
Dr Bee Luan KHOO
-
Workshop on BioFilms on 13th August, 2013:
- Perseverance seminar
room,
Level
5, Enterprise Wing, 1 CREATE WAY, Singapore 138602
-
BioSyM Seminar by Nanyang Asst.Prof.Ali Miserez (School
of Materials Science and Engineering School of Biological Sciences, NTU)
- Genotype to Mechanical Phenotype
Mapping:
Combining
NextGen
Sequencing
with
Proteomics and
Materials
Science
- 18th July 2013, 10.30 am
- Perseverance seminar
room,
Level
5, Enterprise Wing, 1 CREATE WAY, Singapore 138602
-
BioSyM Seminar on "Cell mechanics and the cytoskeleton: Force transduction, nanomechanical properties and in vivo probing of cytoskeletal proteins"
by Dr.Yaron R. Silberber, Research Fellow, The National Institute of Advanced Industrial Science and Technology (AIST), Japan
Friday, 12 July 2013,
2:00 pm – 3:00 pmLocation: Perseverance 2 Conference Room, Level 5, Enterprise building, CREATE Campus, UTown, NUS
Abstract: The living cell is embedded in a complex mechanical environment, in which its behavior is constantly influenced by mechanical cues arriving from the extracellular matrix and from neighboring cells. These signals regulate various cellular processes including differentiation, gene expression, mitosis, development and apoptosis. Hence, understanding the mechanisms that are involved in cellular transduction of forces is crucial for understanding how those forces affect the living cell.
In the first part of my talk, I will present the idea of force transmission through the intracellular cytoskeleton and the novel method we have developed to detect and quantify these forces in vivo. I will then continue to discuss the implications these forces may have on cell behavior and present some experimental ideas, which I have been working on during my research at University College London and Kyoto University in Japan. In addition, I will present the results of regime-dependent nano-mechanical characterization of muscle cells that we have developed for the use in the determination of Young’s modulus of living cells. I will finish the talk with the latest project that I have been working on, which focuses on the development of an antibody-functionalized nanoneedle array for the use in stem cell sorting for medical transplantation. Latest results for the use of a novel label-free, force-based approach for probing intracellular cytoskeletal proteins, in vivo, will also be presented.
Speaker Biography:
Yaron Silberberg is a Research Fellow at the National Institute of Advanced Industrial Science and Technology (AIST) in Japan. Yaron completed his PhD in the field of Bio-Nanotechnology in 2009 at the London Centre for Nanotechnology, University College London (UCL), under the supervision of Prof. Mike Horton and Dr. Andrew Pelling. In his project he investigated the nanomechanical and biological behavior of skin cells using various live-cell imaging techniques combined with Atomic Force Microscopy (AFM) for the manipulation and observation of cells on the nanoscale, a project funded by Unilever and the BBSRC. After obtaining his PhD, he was awarded the Japan Society for the Promotion of Science (JSPS) award and continued his post-doctoral research at Kyoto University, where he focused on live-cell force measurements and on the nanomechanical properties of muscle cells. He later joined the National Institute of Advanced Industrial Science and Technology (AIST) in Japan, where he currently works on a project to develop a novel technique for the use in stem cell sorting for medical transplantation, using antibody-functionalized nanoneedle arrays.
-
organised by Hebrew University of Jerusalem (HUJ), National University of Singapore (NUS) and Singapore - MIT Alliance for Research and Technology (SMART)
-
Workshop on "Cellular Systems Research: from signaling and cell-ECM interactions to cell motion, cell-cell coordination, and tissue formation", on Tuesday, 25th June, 2013,
9:30 am – 4:30 pm, Perseverance Rooms 1 & 2 , CREATE Enterprise Wing Level 5
-
Seminar on "In vitro 3D Heterotypic Cell Culture Assay" by Prof. Seok (sid) Chung, Korea University, on Thursday, 27th June 2013, 11 am - 12 noon, Perseverance Rooms 2 , CREATE Enterprise Wing Level 5
-
Workshop on "Microfluidics and Metastatic Cancer" on Saturday, 29th June 2013, 9 am - 12 noon, Perseverance Rooms 1 & 2, CREATE Enterprise Wing Level 5
-
-
BioSyM Seminar on 6th July 2012 (Friday) @ 3 pm, BioSyM Conference Room, S16-07
- ELECTROCHEMICAL BIOSENSOR DEVELOPMENT FOR STUDIES IN NEUROSCIENCE AND DRUG SCREENING
- Abstract:
Nitrogen derived free radicals, such as nitric oxide (NO), and peroxynitrite (ONOO-) play a central role in normal physiological processes. However, the high reactivity and short-lifetime of these reactive oxygen and nitrogen species make their detection difficult and thus, very sensitive detection methods are in strong demand. Electrochemical biosensors were developed based on biocompatible thin films, nanomaterials, and conducting polymers that have been used either for direct detection or as matrices for immobilizing the biomolecules. Various experiments were performed to characterize the biosensors and immunosensors. The applications of the proposed biosensors were conducted for the determination of target compounds in vitro and in vivo real sample analysis, thus increasing our understanding of the various mechanisms of these reactive nitrogen species (NO, ONOO-) and their protein counterparts (nNOS, iNOS) in the biological environment.
- COMBINATORIAL PHYSICAL MARKERS FOR MULTIPOTENCY
- Abstract:
Mesenchymal stem cells (MSCs) have garnered significant interest from the scientific community owing to their ability to develop into many mature cell types for applications in regenerative medicine. To generate lineage committed MSCs for applications involving adipose, bone, cartilage or muscle regeneration, soluble chemical growth factors are typically supplemented to culture media as part of a differentiation cocktail. In addition to soluble factor signaling, subtle changes to the cell’s local biophysical microenvironment can also have significant effect on whether the cell remain in their immature (multipotent) state or becomes committed to a specific lineage. This often leads to a heterogeneous mixture of MSCs that differ in their stage of commitment and extent of differentiation. The functional heterogeneity of culture expanded MSCs therefore pose a significant challenge to the efficacy and safety of potential MSC-based therapies, as implanted culture expanded MSCs may play out a biology that is different from their intended purpose. This is further complicated by the lack of immunophenotype markers that are indicative of MSC multipotency. In this work, we examined several quantitative features of bone marrow derived MSCs and correlated each mechanical property with their differentiation potential. The parameters include cell size, spread area, elasticity, nuclear to cytoplasmic ratio and relative nuclear fluctuation. Of particular interest is whether any of these physical signatures or combinations thereof, could prospectively identify multilineage subpopulations among different adult and fetal MSCs from precommitted progenitors. We find that among the different mechanical properties screened, only cell elasticity and nuclear fluidity significantly correlate with the differentiation behavior of MSCs. These findings can be recapitulated in vivo, indicating that this MSC classification methodology could be useful in capturing cells that are therapeutically relevant. Together this work establishes a minimal set of physical criteria for defining multipotency and could provide predictive measures for the identification of commitment towards multiple cell types.
-
Seminar on "Small scale rheology for screening scarce materials", 2nd July 2012, 11 am @ BioSyM Conference Room, S16-07, NUS
- Speaker: Prof. Eric M. Furst, Department of Chemical and Biomolecular Engineering and Center for Molecular and Engineering Thermodynamics, University of Delaware, USA
- Abstract: Rheological measurements that characterize the flow properties of a substance can be difficult to perform for many emerging materials, especially when a material is demanding to synthesize or prohibitively expensive. Fortunately, microrheology methods can be used when a material's availability is limited, since it requires small sample volumes, ranging on the order of 1nL-10μL. In addition, microrheology has several complementary features that make it indispensable for characterizing scarce materials, specifically those developed for therapeutic applications. These factors include measurement acquisition times on the order of seconds, a large dynamic frequency response, and exquisite sensitivity to incipient properties. Microrheology uses the motion dispersed micrometer-diameter probe particles to measure the surrounding material's rheology. The technique can be divided into two approaches that are distinguished by the driving force of the probe motion. In the first, active microrheology, the embedded probe particles move in response to an external force, typically generated by optical tweezers or magnetic fields. In the second, passive microrheology, the Brownian or thermal motion of the embedded probe particles is measured and the rheological properties are calculated by the Generalized Stokes–Einstein Relation (GSER). The introduction of passive microrheology has stimulated many of the advances in biomaterial microrheology over the past two decades. In this talk, I will describe recent work on high-throughput viscometry and rheology using a combination of microrheology and microfluidics. A series of microrheology samples is generated as droplets in an immiscible spacer fluid using a microfluidic T-junction. The compositions of the sample droplets are continuously varied over a wide range. Viscosity measurements are made in each droplet using multiple particle tracking microrheology. I will review the key design and operating parameters, including the droplet size, flow rates, rapid fabrication methods and passive microrheology techniques. The combination of microrheology with microfluidics maximizes the number of viscosity measurements while simultaneously minimizing the sample preparation time and amount of material, and should be particularly suited to the characterization of protein solution viscosities of therapeutic agents. I will conclude with an outlook of future work, including active non-linear microrheology and high-throughput rheological fingerprinting.
-
BioSyM Seminar on 8th June 2012 (Friday) @ 3 pm, BioSyM Conference Room, S16-07
- Speaker 1: Dr.Christopher Ochs
- OXYGEN SENSORS FOR MICROFLUIDIC 3D CELL CULTURES
- Abstract:The real-time detection of multiple analyte concentrations (such as O2, CO2, glucose, growth factors) and the monitoring of variations in the physical environment (e.g. temperature, pH, ionic strength) are vital for the understanding of cell growth and proliferation, the communication between cells and their reaction to physical or biological stimuli. Recently, there has been interest in the cultivation of cells in temperature gradients or under mechanical stress, especially to gain information about biochemical signaling pathways between cells, mechanotransduction, and migration behavior in 3D cell cultures. However, up to date, most techniques only allow for the bulk analysis of the extracellular media. Methods exploring the independent and parallel analysis of multiple analytes in complex cell cultures remain to be discovered. A suitable approach will have to present a large number of individually addressable sensors immobilized in direct proximity to the cells, allowing for detection of the local concentration while simultaneously having negligible effect on cell proliferation or the composition of the biological environment. We aim to fabricate a range of cell culture compatible bead-based probes, which can be equipped with sensor molecules depending on the analyte of interest. The beads are rendered biocompatible and functional by applying a suitable layer-by-layer (LbL) coating (e.g., PEG).[3] A microfluidic device custom-built for the control and manipulation of certain biophysical parameters is then loaded with the cells and sensor beads of interest. Using confocal fluorescence microscopy, the obtained signal from the beads can be quantified and changes in cell morphology, viability and migration behavior in response to external stimuli (e.g. drug addition, temperature or oxygen gradient) can be monitored. We also envisage the use of computer models to extrapolate concentrations between data points (= sensor beads) and to calculate a continuous analyte distribution in the cell culture over time. Oxygen is an important factor influencing cell viability, migration, and differentiation; hypoxia has been linked with increased resistance to radiation therapy and certain anticancer drugs. We developed sensor beads based on layer-by-layer (LbL) assembled polyelectrolytes modified with an oxygen-sensitive luminescent dye and demonstrate potential of these biosensors to examine the effect of oxygen gradients in 3D cell cultures. The bead fluorescence intensity in response to various oxygen concentrations is investigated and their cell culture compatibility is evaluated. The oxygen distribution in a novel microfluidic device is simulated for various oxygen gradients and validated experimentally using oxygen sensors beads. Some preliminary results are presented to demonstrate the potential of LbL-assembled oxygen sensors for the measurement of the local oxygen tension around cells.
- Speaker 2: Mr.Kong Tian Fook
- Liquid metal radio-frequency microcoil for magnetic resonance relaxometry
- Abstract: In recent years, labs on a chip (LOC) or micro total analysis systems (mTAS), have been the main sources of innovation for microfluidic technology. The introduction of microsolidics, a fabrication method for multi-layered three-dimensional (3D) metallic structures in microchannels has revolutionized how metallic conductors are integrated in the LOC platform. The key advantage of using liquid-metal technology is the ability of making flexible complex 3D electrical conductors, confined in a microchannel, with only a few fabrication steps. In this talk, we demonstrate a novel technique to fabricate three-dimensional multilayer liquid-metal microcoils together with the microfluidic network by lamination of dry adhesive sheets. The microcoil was characterized for 0.5 T 1H magnetic resonance relaxometry (MRR) measurements. In addition, we used the liquid-metal microcoil to perform a parametric study on the transverse relaxation rate of human red blood cells at different hematocrit levels.
-
BioSyM Seminar on 27th April 2012 (Friday) @ 3 pm, SMART Conference Room, S16-05
- Speaker 1: Dr.Binu Kundukad
- Gelation of the genome by topoisomerase II targeting anticancer drugs
- Abstract: Topoisomerase II regulates the topology of DNA by catalysis of a double strand passage reaction. Inhibition of this reaction prevents cell replication, and, thus, is a pathway targeted by anticancer drugs. The passage reaction was inhibited by AMP-PNP, a non-hydrolyzable analog of ATP, as well as the drug ICRF-193. Microrheology assays showed gelation due to the formation of a self-catenated network of circular DNA molecules. A cell-killing mechanism is proposed by gelation of the genome through TOP2-mediated interlocking of looped DNA segments of the replicated chromosomes.
- A Real-Time Microscopic 3D Factory - Holographic Optical Tweezers with Volume Holographic Microscope
- Abstract: Since their introduction in the 1980s, optical tweezers have permeated research in the biological and physical sciences. Microscopes with optical tweezers are used to observe, move and manipulate small objects like microsphere-based system, and cells. As the experimental complexity grows, the need for multiple trapping arises. Holographic optical tweezers (HOT) utilize a spatial phase modulator to create multiple traps and do real-time 3D manipulation. In SMART, we combined HOT techniques with volume holographic microscope (VHM). VHM captures three-dimensional intensity information on a two-dimensional camera using a volume hologram (VH) in the imaging system. It allows multiple focal planes to be recorded without scanning. With these techniques combined, we can manipulate small objects in 3D space and visualize multifocal planes information simultaneously.
-
BioSyM Seminar on 30th March 2012 (Friday) @ 3 pm, SMART Conference Room, S16-05
- Speaker 1: Dr.Poon Zhiyong
- Therapeutic applications of Mesenchymal Stem Cells
- Abstract: Approaches for the use of mesenchymal stem cell (MSC)-based therapy towards the treatment of degenerative and autoimmune diseases is evolving; however, owing to the functional heterogeneity of culture expanded MSCs, a central challenge has been to classify MSC subpopulations in a way that offers predictable and effective therapeutic results following their use. We demonstrate how microfluidic tools and biophysical cell cytometry aid in this process, and show that MSC subpopulations isolated in this manner are predisposed for different therapeutic applications.
- Speaker 2: Mr.Ng Wei Qing Justin
- How does the amyloid-beta peptide perturb neuronal membrane fluidity? A Microfluidics system approach
- Abstract: The Amyloid-beta peptide (Abeta), is implicated in the pathology of Alzheimer's disease. Neuronal uptake and accumulation of Abeta within the cytoplasm causes the formation of amyloid-plaques, disrupting cellular function and eventually leading to cell death. Abeta also has action at the neuronal membrane through its binding to gangliosides such as GM1, and neuronal receptors such as NMDA and glutamate receptors. Binding of Abeta to the neuronal membrane is thought to perturb membrane fluidity as well as the phase organization of the membrane, resulting in downstream effects such as alteration metabolism pathways, all of which result in altered cellular and membrane function. This study uses a microfluidics approach to recreate a 3D microenvironment where neurons can grow healthily and the environmental factors can be easily manipulated. The effects on the neuronal cell membrane from addition of Abeta is examined via confocal fluorescence-correlation spectroscopy.
-
BioSyM Seminar on 2nd March 2012 (Friday) @ 3 pm, SMART Conference Room, S16-05
- Speaker 1: Dr.Ng Chee Ping
- The Ballad of the Swedish Chef, Mixed Berries and Waffles:
Development of Functional Microwell Arrays for Cytometry of Rare Cells in Bone Marrows and Endometriosis
- Abstract: We are developing functional hydrogel microwell arrays for image cytometry of clinical specimens to study fundamental questions in endometriosis and stem cell biology. In particular, our interests lie in understanding the prevalence and mechanism of colony forming rare cells in endometriosis and bone marrows. Compared to traditional assays where cells have to be plated sparsely on cell culture dishes, the high aspect ratio arrays fabricated from soft photolithography allow the isolation of individual cells or a small group of cells in a more physiologically relevant microenvironment by maintaining their cytokine interactions with their neighbors. In this seminar, I will provide an update on our progress on the development of these microwells, using expanded mesenchymal stem cells for high content characterization and analysis such as well stability and cell behavior. I will also touch on our collaboration with Prof. Han's team on exploring the use of inertial microfluidics to deplete red blood cells from peritoneal and bone marrow samples for downstream studies of nucleated cells. The physical method utilized a spiral shaped device to separate the desired nucleated cells and red blood cells based on their size difference. The feasibility of the device was assessed by analyzing the sorted cells using flow cytometry for enrichment efficiency and screening the cells for the prevalence of colony forming units using traditional plating methods. These technologies may eventually form part of a powerful system diagnostics platform for translational research of complex clinical fluid specimens and also a great recipe for making hearty delicious cornflake waffles with mixed berries.
- The Effect Of Endothelial Dll4 Exosomes On Endothelial Cell Sprouting
- Abstract: Angiogenesis is the formation of new blood vessels from existing vasculature. Delta-like ligand 4 (Dll4), a notch ligand, is known to play an important role in this process by regulating endothelial cell differentiation. When stimulated by a VEGF gradient, endothelial cells differentiate into two distinct cell types, tip and stalk cells. Tip-cells lead the front of a forming sprout, while stalk cells trail behind, supporting the newly formed sprout. Dll4, which is upregulated in tip cells, is known to repress the expression of tip-cell specific genes in the neighbouring cells, hence, allowing tip-stalk cell specification. It has been hypothesized that during Dll4-Notch signalling, following the activation of the Notch receptor, endocytosis of Dll4 leads to its targeting to endosomes and subsequent packaging into exosomes. Exosomes are nanovesicles that have been reported to play a role in cellular communications via their ability to carry biological molecules. Work by Sheldon et al., showed that Dll4 if packaged into exosomes, can affect Notch signalling and promote a tip-cell phenotype. By utilizing microfluidic devices to investigate the effect of endothelial derived exosomes on angiogenic sprouting, our results show that endothelial-derived Dll4-exosomes repress angiogenic sprout formation instead.
-
SMART Biomedical CAREER TALK 2011/2012, by Dr.Thomas Murphy of Research Instruments and Origen Laboratories on 8th Dec 2011 (Thursday), 4 pm @ Seminar Room 1, Centre for Life Sciences (CeLS)
- Dr. Thomas Murphy is a cell biologist with over 10 years’ experience in molecular cell biology and target identification and validation. His particular research interests include biological imaging, cell signaling, and angiogenesis. Prior to joining Research Instruments, Dr. Murphy worked as an Applications Scientist at Thermo Scientific (Dharmacon RNAi Technologies), to advise fellow scientists on advancements being made in the field of RNA interference. During his post-doctoral fellowship, he worked at the Novartis Institute for Functional Genomics (GNF) in San Diego, where he was responsible for conceiving and implementing siRNA-based genomic screens against inflammatory and oncology pathways. He received his B.A. in Biology from Occidental College, followed by his Ph.D. from the Molecular Biology Institute at University of California, Los Angeles. He has also held research positions at UC San Francisco and Amgen Inc. Dr. Murphy is currently Chief Scientific Officer at Research Instruments and heads their Life Science applications team. He is also General Manager of Origen Laboratories, a laboratory service provider which specializes in performing microarray-based research projects for clients.
-
BioSyM Seminar on 28th Nov 2011 (Monday) @ 3 pm, SMART Conference Room, S16-05
- Cell Encapsulation induced angiogenesis on a chip
- Abstract:
Cell-encapsulating alginate beads have the potential to serve as a sustained release system for delivering therapeutic agents in vivo while protecting the cells from the body’s natural immunity response. To date, there is still no representative in vitro model for cell-encapsulation therapy that would provide a suitable platform for quantitative analysis of physiological responses to secreted factors, specifically designed for angiogenesis therapeutics. Here, we introduce a novel angiogenesis-on-a-chip system for the evaluation and quantification of capillary growth from an intact endothelial cell monolayer in response to soluble factors released from human fetal lung fibroblasts encapsulated in beads. We confirmed that cell-encapsulating beads induced an angiogenic response in vitro, demonstrated by a strong correlation between the encapsulated cell density in the beads and the length of the vascular lumen formed in vitro. Cell-encapsulating beads were further shown to exert an angiogenic activity in a subcutaneous mouse model, forming an extensive network of functional luminal structures perfused with red blood cells.
-
BioSyM Seminar on 28th Oct 2011 @ 3 pm, SMART Video Conference Room, S16-05
- From Lipids to their global analysis by mass spectrometry
- Abstract:
When one was to discuss biomolecules, DNA, RNA and proteins usually come to mind. Lipids, on the other way are gravely neglected despite their integral impact on biological systems such as acting as second messengers, homeostasis and maintaining structural integrity. Although still poorly defined, some experts estimate the number of distinct molecular species of the lipidome at 10,000 to 100,000, some of which are already targeted as therapeutic targets. Given the diversity and complexity of lipids, advanced analytical methods (termed lipidomics) are needed to describe them globally, and subsequently address fundamental questions such as lipid function as well as applied biology and biotechnology. In this talk, I will attempt to first discuss the many functions and importance of lipids in biological systems. With that, we will shift focus onto the modern techniques and applications on disease biology with my work on dengue as an example.
-
BioSyM Seminar on 14th Oct 2011 @ BioSyM Meeting room, S16-07
- Speaker: Dr.Hemant Vijaykumar Unadkat, MIRA Research Institute, Department of Tissue Regeneration, University of Twente, The Netherlands
Title: Braille for cells: Cells to phenotypes
Abstract: Evolution in materials processing and synthesis techniques allows us to fabricate and synthesize biomaterials with variable properties and composition. However, present methods for testing a multitude of these biomaterials or their properties are not apt enough except a few. One of the important properties for implantable biomaterials is their surface topography. Unfortunately, nature does not prescribe the optimal surface topography for a given biomedical application, and the number of possible surface patterns that can be created is virtually unlimited, considering that cells are in the order of tens of micrometers whereas patterns can be created at nanometer resolution. The underlying mechanisms defining the interplay of cells with substrates are only partially understood. I’ll discuss the potential of a high throughput screening platform (TopoChip) for screening and directing cell fate by means of surface topographies. The topographies used in the TopoChip are randomly generated using mathematical algorithms. In my talk, I will show that by combining the power of high-throughput screening with mathematical design of micrometer-range surface topographies we can decipher the “Braille code” of cell-topography interactions
-
BioSyM Seminar on 30th Sept 2011 @ 3 pm, SMART Video Conference Room, S16-05
- Speaker 1: Dr.Vijay Raj Singh
- Photon reassignment for structured light microscopy for deep tissue 3D imaging applications
- Abstract: In this talk, a new method will be presented for 3D visualization of biological tissues utilizing structured light wide-field microscopic imaging system. This method provides the capability to image deeper into biological tissue by reassigning fluorescence photons generated from off-focal plane excitation. Recently, novel methods for 3D resolved imaging based on structured light illumination have been developed by several groups (Wilson et al, Gustasson et al, and Mertz et al) allowing wide-field visualization of the focal plane while rejecting out-of-focus background “haze”. While these methods improve image contrast, the loss of out-of-focal plane fluorescent photons limits image signal-to-noise ratio (SNR). Proposed method seeks to better utilize these “lost” photons by using the ‘prior knowledge’ about the optical transfer function of the structured light illumination. Utilizing a maximum likelihood approach, the most likely fluorophores distribution in 3D is identified that produces the observed image stacks under structured and uniform illumination using an iterative maximization algorithm. The proposed model is first validated with the simulation data where the results show the substantial improvement in SNR compare to existing methods and then it further applied for experimentally recorded data. Results show the significant optical sectioning capability of tissue sample while preserving the photons count, which is usually not achievable with other existing structured light imaging methods.
- Speaker 2: Dr.Du Ning
- Compression and self entanglement of single DNA molecules under electric field
- Abstract: The use of electric fields to transport DNA has become an essential technique for a variety of research areas including molecular biology, gene therapy, and fundamental studies of polyelectrolytes. In most applications, non-linear electrokinetics due to the interplay of negatively charged DNA and the electric field are neglected. Here we experimentally study the effects of a uniform electric field on the conformation of fluorescently labeled single DNA molecules. We demonstrate that a moderate electric field (∼200 V/cm) strongly compresses isolated DNA polymer coils into isotropic globules. Insight into the nature of these compressed states is gained by following the expansion of the molecules back to equilibrium after halting the electric field. We observe two distinct types of expansion modes: a continuous molecular expansion analogous to a compressed spring expanding, and a much slower expansion characterized by two long-lived metastable states. Fluorescence microscopy and stretching experiments reveal that the metastable states are the result of intramolecular self-entanglements induced by the electric field. These results have broad importance in DNA separations and single molecule genomics, polymer rheology, and DNA-based nanofabrication.
-
SMART BioSym Seminar on Tuesday, August 23, 2011, 1:30 pm
Speaker: Ki Hean Kim, Ph.D., Assistant Professor in Mechanical Engineering and Integrative Biosciences and Biotechnology Pohang University of Science and Technology (POSTECH), Pohang, Korea
Title: Polarization-sensitive optical coherence tomography: its clinical applications and development
Abstract: Polarization-sensitive optical coherence tomography (PS-OCT) is a 3D tissue imaging technique which provides information of structure and birefringence. PS-OCT can be used as non-invasive optical tissue biopsy down to a few millimeters. In this talk, clinical studies and technological development of PS-OCT will be presented. Clinical studies are human vocal fold and human burn imaging. Technological development is a new PS-OCT method, which is based on depolarized light and frequency multiplexing, to increase its functionality.
-
-
BioSyM Monthly Seminar on 25th May 2011 @ 8 pm, SMART Video Conference Room, S16-05
- Speaker 1: Ali Asgar Bhagat
- Microfluidic technologies for circulating tumor cell (CTCs) isolation
- Speaker 2: Li Jie
- Molecular mechanism of activation of the EGFR kinase studied using umbrella sampling and transition path sampling
-
BioSyM Monthly Seminar on 27th April 2011 @ 8 pm, SMART Video Conference Room, S16-05
- Speaker 1: Dipanjan Bhattacharya
- Development of Digital Scanned Laser Sheet Microscope for 3D live tissue imaging
- Speaker 2: Sharon Ong
- Automated Tracking of Cells and Conduits from Time-Lapse Confocal Microscopy Images
BioSyM graduate students Seminar (BioGraSS) on 29th March 2011 @ 12.30 pm, BioSyM Conference Room
- Speaker 1: Guofeng Guan
- Deformabillity-based Cytometry With Active Control of Microfluidic Channels
- Speaker 2: Tan Chin Wen
- Stem cells in endometriosis
BioSyM graduate students Seminar (BioGraSS) on 18th Feb 2011 @ 12.30 pm, BioSyM Conference Room
- Speaker 1: Sei Hien Lim
- Induction of Angiogenesis in Microfluidic Devices using Prolyl Hydroxylase Inhibitors
- Speaker 2: Pradeep Anand Ravindranath
- Robust inhibition of Hepatitis C viral propagation
BioSyM Monthly Seminar on 16th Feb 2011 @ 8 am, SMART Video Conference Room, S16-05
- Speaker 1: Young Kum Park
- Stem Cell Differentiation within 3D Mmicrofluidic Environment
- Speaker 2: Hoi Siew Kit
- Optical Trapping and its Applications in Microfluidics
SMART BioSyM Invited Seminar: 8 Feb 2011 @ SMART S16 Level 5 Conference Room, 4 - 5 pm
- Title: Infection Treatment Vaccination as a Model of Protection Against Malaria
- Speaker: Jingyang Chen
- Jingyang Chen is a biologist in the Laboratory of Malaria Immunology and Vaccinology (LMIV) at the National Institute of Allergy and Infectious Diseases (NIAID) in the United States. He currently works on non-human primate models of malaria and second generation sequencing of clinical samples from longitudinal studies in Tanzania and Mali.
-
BioSyM graduate students Seminar (BioGraSS) on 13th Jan 2011 @ 12.30 pm, BioSyM Conference Room
- Speaker 1: Yuchun Liu
- Vascularised bone tissue engineering using human umbilical cord blood derived endothelial progenitor cells (EPC) and fetal bone marrow-mesenchymal stem cells (MSC)
- Speaker 2: Wong Cheng Lee
- High throughput sorting of cells into different phases of the cell cycle using inertial microfluidics
-
-
-
-
5-31 July 2010 - Summer School on "Cell and Molecular Mechanics in BioMedicine with a focus on Mechanobiology" http://www.dbs.nus.edu.sg/mechano/events/index.html
-
30-31 July 2010 - BioSyM Summer Workshop & Scientific Advisory Board Meeting
-
1-6 August, 2010 – World Congress of Biomechanics
Symposium on Biological Systems and Mechanics @ WCB 2010, Organized by: Singapore - MIT Alliance for Research and Technology Chair: Jongyoon Han (MIT) and Peter So (MIT)
|
 |